|
Soil and water are vital to life on this planet. We must protect these resources and use them wisely—our survival as a species depends on them. Despite recent impressive strides in improving the environment, evidence is overwhelming that more effective action must be taken to address such critical issues as acid rain, hazardous waste disposal, hazardous waste landfills, and groundwater contamination. It is also vital that we realistically assess the potential health and environmental impacts of emerging chemical products and technologies. The problems are certainly complex and demand a broad array of new research initiatives in chemical engineering. Technological activities—of which chemical manufacture and processing are key parts—begin with the extraction of raw materials from the environment. These then proceed through numerous steps—processing, storage, handling, transportation, and use—and finally end with the ultimate return of processed materials or their residues to the environment. The figure below illustrates the lifecycle of a generic chemical compound. |
|
A simplified picture of the life cycle of
chemicals in the environment. (Click on image for larger
view.)
|
|
The redistribution of chemicals within the environment may have adverse impacts. Harmful compounds of certain elements (e.g., nitrogen, sulfur, chlorine, fluorine) may be widely mobilized, that is, transported through the soil by the action of subterranean groundwater. Other elements may be converted from innocuous forms (e.g., mercuric sulfide in cinnabar) to highly toxic forms (e.g., methyl mercury). And some elements, notably heavy metals such as selenium, and chromium, are now known to be highly toxic in their elemental form. Since chemical engineers are involved in all aspects of chemical manufacture and processing it is only appropriate that it should be their responsibility to safely manage chemicals in the environment. An essential ingredient in our approach to environmental and safety problems has been the change from reactive to proactive: in other words, instead of always responding to crises and public pressures, we have begun anticipate and prevent problems. Reactive measures cannot be avoided, of course, since it is up to the current generations to take care of environmental problems created during the last century by varying degrees of ignorance, apathy, and greed. Nevertheless, it is essential that we turn our attention to the future, and chemical engineers have taken a lead role in these prevention endeavors. To deal with these new challenges it is necessary to acquire and implement a detailed understanding of the chemical and physics of processes at the molecular level. Such basic understanding is crucial for the design of plants that are safer and cause less pollution, the development of better ways to manage and detoxify hazardous waste, and the prediction of the fate of chemicals in the environment as well as the effects of chemicals on humans and ecosystems. It's recognized that human activities can affect environmental quality and human health. Introduction of new chemical products, adoption of different technologies, or changes in resource utilization can lead to emissions that affect the physical, chemical, or biological responses of the receiving ecosystems. This can, in turn, affect human beings and other resources. A risk assessment of the potential health and welfare effects of such changes can indicate whether and to what extent mitigation measures should be taken, or original decisions rethought. |
|
Through acts of carelessness, miscommunication, negligence, or ignorance, humans have had a significant impact on ecological systems. Often, this impact is the result of continuous, low-level interaction between human habitation and the surrounding environment. On occasion, however, humans have accidentally released very dangerous compounds. The effects of human technology on the environment span scales ranging from site-specific (as when a chemical spill occurs) to global (as when waste is dumped in the world's oceans). Some examples of well known environmental issues and their scale are shown in the table below.
These issues have arisen for a variety of reasons, from major accidents at chemical plants (Seveso and Bhopal), to regional and global impacts associated with energy utilization, the improper disposal of chemical waste (Love Canal and Times Beach), and chemicals that have dispersed and bioaccumulated affecting wildlife (selenium and DDT) and human health (cadmium, mercury, PCBs, and asbestos). A consequence of these ongoing issues is that much of the public has come to believe that almost all chemicals are hazardous and that nothing is being done to ensure its safety. It is a generally held belief that industry does not deal openly with the public and is quick to cover up problems that arise. These factors have led to a public attitude towards chemical exposure that can best be described as "zero risk." However, it's recognized by not only engineers and scientists but also the legal community that, "In the crowded conditions of modern life, even the most careful person cannot avoid creating some risks and accepting others. What one must not do, and what I think a careful person tries not to do, is create a risk that is substantial."1 The question is, of course, what is a substantial risk? How safe is safe enough? These questions trouble the public, industry, and regulators alike. Scientific understanding and the available data are inadequate to evaluate the true risk to individual safety, or the true risk of damage to human health of the environment, from the exposure to most chemicals or to chemical plant or disposal operations. Laws have been passed and regulations have been developed that require government approval for production of new chemicals, design and operation of chemical plants, workplace exposures, certain product uses, quantities (total amount) and concentrations (amount per total volume) of chemicals in effluent streams, and disposal of waste and byproduct streams. But because of the uncertainties that exist, some regulations have been written to protect against the effects of extremely unlikely worst-case scenarios, resulting in a misallocation of resources, reduced technical innovation, and excessive costs. At the same time, other, less visible hazards that might be the focus of appropriate regulation have been overlooked. A number of major environmental and social costs result from our laws and regulations, including industry safety, power generation, and hazardous waste management. Industry Safety Nevertheless, accidents and unintended chemical releases pose serious financial risks to the chemical and petrochemical industry. In 1984, for example, there were five major accidents in the hydrocarbon-chemical industries, totaling an estimated loss of $268 million. And while that was a particularly bad year, hundreds of lesser accidents occur annually. The total annual cost to the industry of accidents and unintended chemical releases is difficult to quantify. It includes significant costs owing to interruption of business as well as major liability and litigation costs associated with injuries, deaths, property damage, and insurance premiums. It also includes losses of product and feedstock that are direct profit losses for the manufacturer. Costs associated with increased government regulation are also difficult to quantify. Public concern in response to chemical release accidents affect regulators and community policy groups. It is evident that the U.S. chemical industry is already spending large amounts of money to avoid accidents and to deal with their consequences when they occur. These costs are borne, in part, by consumers. Continued expenditures are likely as industry strives to achieve an acceptable level of public safety throughout all chemical industry operations. Combustion of Fuels Many of the ramifications of these pollutants directly affect the atmosphere—issues such as haze, smog, volatile toxic compounds, and global climate change, and we'll examine these in the next topic. There are a number of links between the atmosphere and land and water that are also directly affected by the formation of pollutants during combustion. For example, nitrogen oxides (known collectively as NOx and pronounced 'knocks') and sulfur oxides (known as SOx and pronounced 'socks') are gases issuing from smokestacks, vehicle exhausts, and even backyard barbecues, and these readily dissolve in cloud water droplets. Once dissolved, these chemical species undergo reactions that convert them to sulfuric acid (H2SO4) and nitric acid (HNO3), with the result that, when the cloud droplets grow large enough to rain out, they're acidic rather than pure water. This so-called acid rain is a very serious phenomena in the western world, particularly the United States. The figure below shows the average pH of the rain that fell in the U.S. and Canada in 1980. |
|||||||||||||
|
The annual mean value of pH (a measure of
acidity) in the precipitation that fell in 1980. When pH = 7
it means that the water is completely neutral, neither
acidic nor basic; the lower the pH the more acidic the
water. (Click on image for larger view.)
|
|
Acid rain is very much a regional phenomenon. The intensity of the effect is closely tied to industrial centers where significant amounts of fossil fuels, coal in particular, are burned. However, because the precursors, NOx and SOx are gases and easily transported in the atmosphere by the prevailing winds, acid rain often winds up in places away from the initial combustion sources. This continues to be a very sore point between the United States and Canada, because despite the U.S.'s initial denials, the acid rain that has been lowering the pH of streams, rivers, and lakes in Canada is due in large part to combustion on the U.S. side of the border. There are two obvious solutions to this particular problem: treat the products of combustion so that the only compounds released to the atmosphere are carbon dioxide, water vapor, nitrogen, and oxygen, or clean up the fuel before combustion. The first route is difficult because up to the 1980's stationary power plants didn't have any significant emission controls, and the cost of retrofitting thousands of plants is exorbitant. And the cost of adding these emission controls to the construction of a new power plant adds from 15 to 35% of the total capital cost. The commensurately high cost of cleaning fuel before burning has led chemical engineers to look at a third route for reduced emissions, the combustion process itself. It may be possible to develop clean, fuel-efficient combustion processes as well as to design more economical processes for fuel cleaning prior to combustion. or removing residuals from postcombustion gases. Hazardous Waste Management A variety of methods have been used over the past 100 years to bury hazardous waste. Many of these burial sites now pose a threat to the health of nearby residents and, more broadly, to the nation's underground water supply. A number of these techniques are shown in the cartoon below. The main problem common to each disposal technique is that the waste escapes the confines of the intended burial site. This can occur because of leakage from supposedly leak-proof containers, or leakage through a supposedly impermeable barrier such as the bottom of a disposal pond or a layer of deep underground shale. Once the waste gets outside the burial zone, it can often be readily transported by underground aquifers. Sometimes the presence of these underground "rivers" escapes the surveyors locating the disposal site, and sometimes the aquifers are seasonal, only forming due to regional rainfall. And sometimes, unfortunately, in the past waste was purposely put down as deep as possible, based on the idea that if it was deep enough it couldn't come back to haunt anyone. |
|
Past methods of solid and liquid waste disposal
threaten water supplies today. The arrows denote transport
of water and waste through permeable soil and rock. (Click
on image for larger view.)
|
|
It's not just the past burial of industrial waste that causes problems today, however. Studies in California have shown that there are widespread threats to groundwater in California's Silicon Valley; Santa Clara County leads the nation in the number of sites on the National Priority List, most of which are associated with the electronics manufacturing industry. It is estimated that there are over 10,000 sites nationwide that belong on the National Priority List of toxic waste dumps. In the 1980's Congressional legislation brought the Superfund Program into being. The original program viewed cleanup of hazardous waste sites as a relatively short-term program and anticipated that existing waste could be contained for several decades by methods such as building slurry walls and clay caps around the waste to eliminate leakage of the buried material into subsurface waters. After a few years of pursuing such methods it became clear that they did not provide a realistic solution of the problem of containment of waste in existing landfills. Even with the most careful planning and execution, slurry walls leaked and clay caps cracked. It also became clear in the Superfund Program that it would take significantly longer to clean up (i.e., contain) existing waste sites that originally anticipated. When the Superfund Program was reauthorized in the mid-1980's, there was a major shift from existing containment methods towards the study of new technologies to decontaminate soil and groundwater, and to provide new methods for long-term containment of the wastes that could not be readily decontaminated. Chemical engineers have taken a lead role in technology development in the area of waste minimization and treatment. |
|
There are two principal facets of chemical engineering and the environment at the beginning of the new millennium. The first is the application of fundamental knowledge to the design of inherently safer and less polluting plants and processes. The second is the application of fundamental knowledge to the remediation of existing waste. Both of these activities are equally important because with one we address critical problems of the past (and now present) and with the other we prevent these same type of problems arising in the future. Few basic decisions affect hazard potential or have more of an impact on the environment than the initial choice of technology. Thus, when designing chemical manufacturing processes, it is important to select sequences of chemical reactions that avoid the use of hazardous feedstocks and the generation of hazardous chemical intermediates. It is necessary to find reaction conditions tolerant of transient (temporary) excursions in temperature, pressure, or concentration of chemicals and to use safe solvents when extracting reaction products during purification steps. Finally, it is important to minimize storage and in-process inventories of hazardous substances. The term "inherently safer plants" has been used to describe this approach. One further consideration the design of a new process or plant is whether it is going to generate polluting effluents or hazardous wastes. Good design should result in waste minimization in a manufacturing process or plant. Traditional analyses of process economics might show that inherently safer and less polluting plants are less efficient in terms of energy or raw materials usage. Indeed, chemical plants have been designed in the past principally to maximize reliability, product quality, and profitability. Such issues as chronic emissions, waste disposal, and process safety have often been treated as secondary factors. It has become clear, however, that these considerations are as important as the others and must be addressed during the earliest design stages of the plant. This is due, in part, to a more realistic calculation of the economics of building and operating a plant. When potential saving from reduced accident frequency, avoidance of generating hazardous waste that must be disposed of, and decreased potential liability are taken into consideration, inherently safer and less polluting plants may prove to cost less overall to build and operate. And in any case, if the American public is not convinced that chemical plants are designed to be safe and environmentally benign, then the fact that they operate economically will be of little consequence to the public's decision on whether to allow their construction and operation. The chemical reaction pathways chosen for a manufacturing process profoundly influence chemical plant safety because they determine the nature and amounts of all raw materials, solvents, intermediates, and products, and implicitly govern the design and operation of all hardware. The ultimate goal of chemical engineering research to provide inherently safer plants should be to elucidate the connection between process pathways and plant safety and to translate this connection into a quantitative form amenable to engineering design calculations. The array of chemicals, reactions, processes, and types of physical equipment used in industry is exceedingly diverse and constantly evolving. To effectively address the need to inherently safer plants, chemical engineering research is focused on fundamental issues that span the entire range of processing activities, from uncovering detailed chemical reaction mechanisms (the individual steps that occur in a sequence of reactions) to understanding and predicting the gross response of coupled equipment. The goal of such fundamental research is to develop the tools needed to define, discern, and assess the safety issues associated with a given process design and its alternatives. New approaches to the design of commercial chemical syntheses are also being aggressively pursued. A common approach to such designs are through the use of what is called a chemical synthesis tree graph. In this type of graph, the high-value product is shown at the apex, lower-value raw materials are at the base, and reaction steps are shown as nodes connecting all branches within the tree, from raw material to finished product. By showing all possible branches leading to the desired product, it allows the chemical engineer to quantitatively assess the feasibility, economics, and safety of the possible synthesis routes. When the storage and handling of chemical intermediates are taken into account, it is possible to make informed decisions about the optimal synthesis route. As an example, a U.S. chemical plant in Bhopal, India was manufacturing a chemical called Carbaryl, and in the process used at the time it was necessary to store large quantities of a highly reactive intermediate, methyl isocyanate. An accident in the Bhopal plant resulted in the release of large quantities of methyl isocyanate, which had an extremely negative impact on the surrounding population. Had a more expensive, straight-through reaction scheme been implemented, there would have been a negligible inventory of this compound on hand. An important lesson learned from serious accidents such as this is that developing the methodology of using process safety and environmental factors in synthesis tree graphs can provide a better framework for future plant design. Most accidents in chemical plants occur when the plant is not operating at a steady state—for example, when it is starting up or shutting down, or when a temporary (transient) spike in temperature, pressure, or reactant concentration occurs. Fundamental research in non-state-state process control and the management of process transients is therefore of utmost importance to chemical engineers. Design methodology poses a related research issue. It is obviously easier for the designer of a plant of individual process unit to envision how the equipment will operate during the normal production mode than to envision how it will operate under a host of potential scenarios that arise from process transients. (What happens if the temperature of the mixture in the reactor suddenly goes up because a cooling water pump broke down? What happens if a pressure valve fails and the pressure in a pipeline suddenly doubles? What happens to your pumping system if a heater in a chamber fails and the liquid mixture suddenly solidifies? What happens when your raw material is suddenly twice as rich in the critical active ingredient?) The safety of chemical plants and reactors are being improved because designers now have the means to envision the complete reaction topography and to assess the consequences of straying from normal operations. This involves the continual development of design tools that incorporate chemical pathway information more systematically into classic chemical engineering design methods for all types of equipment used in plants and processes. |
|
Many environmental issues relating to soil and water, from localized, site-specific problems to global change, are intimately tied to combustion. There are many different types of emissions and sources that have a deleterious effect on our soil and water, but we focus here on combustion because its effect is significant and it illustrates the progress we're making to resolve environmental issues through sustained research into the physical and chemical processes underlying combustion. Specifically, we want to examine the link between NOx and SOx formation and the presence of acid rain. Naturally, one way to eliminate environmental problems associated with combustion is to cease the burning of carbon-based materials. For better or worse, however, the world today is firmly entrenched in the use of petroleum, coal, and wood as energy sources, and it's highly unlikely that this will change until supplies of these materials become significantly depleted. Even ignoring energy production, our transportation system is strongly dependent on petroleum. And despite the best efforts of the Department of Energy laboratories and the automobile industry, we're not yet at a point where some form of petroleum distillate is no longer required; the technology for all-electric or hydrogen-powered vehicles is not far enough along to make these feasible. The burning of fuel in a practical combustion system, such as a power plant boiler or the cylinder of an internal combustion engine, is at first glance very simple: a mixture of hydrocarbon and air is ignited and burned to carbon dioxide and water. One closer examination this burning turns out to be one of the most complex processes in all engineering. For example, the combustion of the simplest hydrocarbon fuel, methane (CH4), involves more than 50 chemical reactions and over 20 chemical species. This means that the act of burning CH4 in air (79% N2, 21% O2) to form CO2 and H2O involves more than 50 parallel and sequential reaction steps, and produces more than 15 additional chemical compounds. Some of these compounds are stable (long lived), e.g., acetaldehyde (CH3CHO), methanol (CH3OH), and carbon monoxide (CO), while others only survive in the vicinity of the flame (e.g., hydroxyl radical, methyl radical). The point is, the combustion of a very simple fuel yields undesired compounds that include alcohols, acids (albeit weak ones), aldehydes, and ketones. These undesirable chemicals are in addition to the NOx inadvertently produced from high temperature nitrogen oxidation reactions. During the past five decades, major progress has been made in developing a mechanistic understanding of the combustion of methane (CH4), ethane (C2H6), ethylene (C2H4), acetylene (C2H2), propane (C3H8), and butane (C4H10). Rates of many individual reactions in combustion have been measured and a good number of those not measured can be estimated from fundamental thermodynamics and chemical kinetics theory. The point is, after five decades there is some degree of confidence that we understand how smaller hydrocarbons burn (methane through butane), but we're still a long way from detailed understanding of how the heavier hydrocarbons combust. An understanding of how a complicated mixture of hydrocarbons burn (e.g., gasoline) is even further away. Other major gaps in our understanding concern the reactions that lead to the formation of polycyclic aromatic hydrocarbon (PAHs) and soot, as well as the oxidation chemistry of other elements besides carbon and hydrogen that may be present during combustion, including nitrogen, sulfur, and halogens (chlorine, fluorine, bromine, and iodine). These latter issues are in the forefront of current chemical engineering combustion research. |
|
Possible chemical pathway leading to the
formation of large polycyclic aromatic hydrocarbon (PAHs)
during combustion of a simple fuel. (Click on image for
larger view.)
|
|
Nitrogen oxide (NOx) emissions from furnaces and boilers comes mostly from oxidation of the nitrogen atoms in the fuel itself; this form is known as bound nitrogen. (As much as we'd like the fuel to be composed of nothing but carbon and possibly hydrogen, we're often stuck with the presence of nitrogen, sulfur, metals, and halogens. These are often present in minute concentrations, but when enough fuel is burned the total quantity of these that are emitted can be large.) In contrast, in internal combustion engines, the NOx emissions are derived largely from oxidation of atmospheric nitrogen. Burners of advanced design currently reduce emissions of nitrogen oxides by a factor of 2 to 3 from uncontrolled combustion systems by staging the addition of oxygen to produce an initially fuel-rich regime in which the bound nitrogen is partially converted to N2. Potentially greater reduction in nitrogen oxides can be attained by adding additional hydrocarbons downstream of the fuel. This post-flame addition of extra hydrocarbon is called reburning. An example of a low-NOx combustion device is shown in the figure below. |
|
A schematic diagram of a low-NOx
pulverized coal burner. The addition of oxygen is staged to
produce an initial fuel-rich zone in the burner, resulting
in reduced formation of the nitrogen oxides. (Click on image
for larger view.)
|
|
The development of staged combustors such as that shown above for the control of nitrogen oxides is constrained partly by the formation of PAHs and soot. It is also constrained by the partial oxidation of the carbon in the fuel, leading to the formation of carbon monoxide rather than complete oxidation to form carbon dioxide. Both of these undesired effects are due to the use of the fuel-rich zone in the burner. PAHs are of interest because they are now known to be potential carcinogens whose biological activity depends strongly on their molecular structure. It is believed that PAHs form under locally fuel-rich conditions by the successive addition of small hydrocarbon species to aromatic rings, as shown in a figure above. (Benzene, C6H6, is the simplest aromatic compound: it contains six carbon atoms in a flat, hexagonal ring configuration.) On the other hand, a staged combustor cannot be operated on too lean (i.e., oxygen-rich) a fuel mixture because the formation of nitrogen oxides is favored under these conditions. Research into optimal burner design continues in an effort to simultaneously minimize the formation of nitrogen oxides, PAHs, and carbon monoxide. The U.S. uses a disproportionate amount of gas and oil for energy generation compared to coal. It has long been known that this country must increase the fraction of its energy that is derived from coal if it is to be independent of foreign energy sources. The major obstacles to the wider use of coal are environmental. The mineral content of U.S. coals averages about 10% by weight, and sulfur content varies widely (depending on the source of the coal) around an average of approximately 2.5%. The conversion of minerals in the coal to ask particles will be discussed in the next topic, but the conversion of sulfur to sulfur oxides, principally sulfur dioxide (SO2), is of direct consequence to acid precipitation. Current strategies for reducing sulfur oxide emissions from coal-fired combustors are based on the addition of calcium minerals to the combustion chamber. The sulfur in the fuel is attracted to the calcium sorbent materials, and the sulfur is removed from the combustion gas stream as either calcium sulfide or calcium sulfate, depending on the specifics of the combustion environment. The important thing is that the sulfur initially present in the coal doesn't leave the combustor as a sulfur oxide. An example of a low-SOx combustor is shown below. |
|
An example of a coal-fired combustor designed
for reduced sulfur oxide emissions. The calcium adsorbent is
typically limestone. The sulfur in the fuel is bound to the
limestone, which is removed with the ash from the combustion
product gas by a cyclone separator. (Click on image for
larger view.)
|
|
One significant problem with this approach is that the efficiency of sulfur removal is very low; currently, a fivefold excess of calcium sorbent must be used to remove the sulfur. Thus, there is a large volume of spent (used) sorbent to dispose of. This material is not hazardous, but at the very least constitutes one more thing that must be land-filled. An ongoing area of research is in the design of sorbents. It's generally agreed that this basic approach for sulfur removal is sound, so there may be a route (or routes) for major innovation in the design of sorbents for sulfur capture in combustors by tailoring their physical and chemical properties. The key characteristics of an ideal sorbent are large surface area (for maximal sulfur capture), mechanical strength, and fast, complete utilization. Ideally, the sorbent will be regenerable or usable as a byproduct. |
|
There are three distinct problems that exist in hazardous waste management: (1) reduction in the generation of waste; (2) disposal of generated waste; and (3) remediation of old, abandoned waste disposal sites. The problems of handling and disposing of radioactive waste are large the concern of nuclear engineers, often working with chemical engineers to develop separation and encapsulation technologies for radioactive nuclides, and are not discussed here. The most basic way to deal with the continuing generation of hazardous waste is to accumulate, encapsulate, and store only as a temporary measure and to develop new approaches to reduce the volumes generated and to concentrate hazardous waste components or convert them into nonhazardous materials. Abandoned waste sites are remediated by cleaning them up or containing them before they contaminate groundwater supplies. The establishment of priorities for site cleanup and the development of appropriate detoxification technologies require an understanding of the processes by which the waste can migrate or be transformed in the natural environment. The development of a fundamental understanding of the behavior of toxic chemicals in soil and aquatic environments and of possible mechanisms for destroying toxic chemicals has lagged far behind the rapidly unfolding problems surrounding disposal. An cartoon illustrating the fate of toxic chemicals is shown in the figure below. |
|
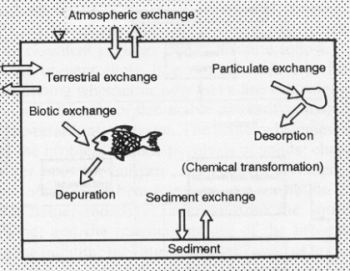 Transport and transformation of toxic chemicals
in soil environments (top) and water environments (bottom).
|
|
Nevertheless, the ability of American manufacturing industries to remain internationally competitive depends on this degradation ability. Engineers in industry did not anticipate that the problems associated with hazardous waste disposal would emerge so rapidly or that destruction and disposal processes would be so difficult to develop. A major effort has been initiated to conduct advanced research and to educate production engineers to solve problems associated with the disposal and environmental behavior of toxic chemicals. Many technologies have been proposed for detoxifying waste by processes that break chemical bonds, thereby converting the original compound to other, benign materials. Among these various methods, thermal destruction, biodegradation, separation processes, and wet oxidation have shown great promise. We'll briefly consider each of these, discussing their strengths and weaknesses, and pointing out ongoing research issues (i.e., unanswered questions). Thermal destruction of toxics The most important, and controversial, technique for the thermal destruction of waste is incineration, where the energy required for destruction is provided by burning the waste, sometimes with the addition of a fossil fuel to ensure high temperatures. The major concern with thermal destruction techniques in general, and incineration in particular, is whether the products from the process—either trace amounts of unreacted waste or compounds unintentionally synthesized from the waste at high temperature—will pose a health hazard. The physical and chemical processes involved in incineration are poorly understood, in part, because there are a number of important variables that determine operating behavior. Factors that influence the destruction efficiency in waste incineration include local temperatures and gas composition, residence time (how long the waste is exposed to high temperature), extent of atomization (breakup into droplets) of liquid wastes, fluctuations in the waste stream composition, combustion aerodynamics, and turbulent mixing rates (how well the waste mixes with oxygen during burning). Even the total destruction of the original hazardous material is not an adequate measure of the performance of an incinerator. Products of incomplete combustion can be as toxic as, or even more toxic than, the materials from which they evolve. Indeed, as discussed earlier, highly mutagenic PAHs are readily generated along with soot in fuel-rich regions of most hydrocarbon flames. Formation of dioxins in the combustion of chlorinated hydrocarbons has been reported. It is essential that we understand the entire sequence of reactions involved in incineration to assess the effectiveness and risks of hazardous waste incineration. Routine monitoring of every hazardous constituent of the effluent gases of operating incinerators is not yet possible. The Environmental Protection Agency (EPA) has established procedures to characterize incinerator performance in terms of the destruction of selected components of the anticipated incoming waste stream. These compounds, called principal organic hazardous components (POHCs) are currently ranked on the basis of their difficulty of incineration and their concentration in the inlet waste stream. The destruction efficiency of an incinerator is expressed in terms of elimination of the test compounds, with greater than 99.99% removal typically judged acceptable, provided that toxic byproducts are not generated in the process. The effectiveness of incineration is most commonly estimated from the heat value of the fuel—the amount of energy released when a specific mass of fuel is burned completely—but this parameter has little to do with the rate or mechanism of destruction. Alternative means of assessing the effectiveness of incineration destruction of various constituents of a hazardous waste stream have been proposed, such as methods based on the rate of thermal decomposition of constituents or the susceptibility of individual constituents to chemical attack. Laboratory studies of waste incineration have demonstrated that no single ranking procedure is appropriate for all incinerator conditions. For example, acceptably low levels of some test compound, such as methyl chloride, have proved difficult to achieve because these compounds are formed in the flame from other chemical species. Therefore, rather than focusing on specific incineration technologies, chemical engineers have been addressing fundamental physical and chemical process common to many of the possible incineration systems through (1) studies of the reaction kinetics (rates) of selected waste materials and (2) the behavior of waste mixtures—solid, liquid, and dispersed—in the incineration environment. Much of what is known to date is based on the past 50 years of combustion research, and this has focused almost exclusively on processes occurring in the flame, where heat release rates and reactive species concentrations are both high. A key issue in incineration chemistry involves understanding the late stages of degradation of waste materials, where temperatures and reactive species concentrations are lower than in the flame zone. Moreover, it has long been known that the introduction of halogen atoms (chlorine, bromine, etc.) into a flame can interfere with combustion by removing important reactive species from the gas mixture. This is a serious issue in understanding waste incineration because many waste materials are halogenated. Studies of the incineration of liquid and solid wastes must determine the rates at which hazardous compounds are released into the vapor phase or are transformed in the condensed phase, particularly when the hazardous materials make up a small fraction of the liquid burned. Chemical engineers are particularly concerned with understanding the effects of the major composition and property variations that might be encountered in waste incinerator operations, for example, fluctuations in the type of waste or in water content, as well as phase separations (possible separation of the material into multiple phases, such as gas and liquid or liquid and solid). Biodegradation The controlled use of biological systems or their products to bring about chemical or physical change is particularly attractive when dealing with dilute waste streams. Biological systems thrive in dilute aqueous media, where they can effectively degrade organic pollutants, absorb heavy metal ions, or change the state of heavy metals ion so they can be bound to other elements and subsequently removed. Biodegradation is be actively pursued as a means of treating as-produced waste streams as well as remediating existing sites where toxic waste has been disposed. Some examples of the use of microorganisms for treating process waste is shown in the table below.
Microorganisms and biological agents can carry out reactions with great chemical specificity and efficiency, and genetic engineering provides means for developing strains of organisms and classes of enzymes with nearly unlimited capabilities for effecting desired chemical changes. In addition, many microbial systems have high affinity for metals ions, and metal ions are often moved from an aqueous solution into the biological cell through active transport. Accordingly, such reactions as the biological reduction of a heavy metal ion can be carried out at relative fast rates, although at very small concentrations of the metal. There are significant research issues under investigation by chemical engineers in the design and optimization of bioreactors for dilute waste stream treatment, including the design of efficient contactors, the use of immobolized cells in reactors, and the explanation of the mass transport processes and reaction kinetics. Related research areas in chemical engineering include the formulation of biocatalysts, the development of bioseparations, and the use of chemical engineering expertise in process control and optimization to better understand the behavior of large microbial populations.
The promise of biological treatment of heavy metal ions has already been illustrated by strains of microorganisms that tolerate mercury, chromium, and nickel—heavy metals that are generally toxic to microorganisms. Tolerant microorganisms have been isolated through classical adapation and strain-selection studies rather than by recombinant DNA techniques. Mercury-tolerant microorganisms have been shown to possess an enzyme that is not present in the nonresistant strains. This enzyme, mercuric reductase, is able to catalyze the reduction of mercury(II) ions to metallic mercury. (Mercury disolved in the aqueous phase is converted to its elemental liquid state, upon which it settles out of solution.) Since the mercury(II) ion is the toxic species and metallic mercury is chemically inactive, the microorganism is able to detoxify a solution that contains mercury(II) ions. |
|||||||||||||||||||||||||||||
|
A laboratory-scale system for the treatment of
2,4,6-trichlorophenol. The first reactor is packed with
nylon beads coated with the appropriate microorganisms, and
this immobilized cell system is operated anaerobically
(oxygen-free). The bacteria partially dechlorinate the waste
before it is passed on to a second reactor for complete
degradation.
|
|
Microorganisms hold tremendous promise for improvements in the treatment of hazardous waste, but genetically altered microorganisms present both regulatory bodies and industry the complex task of identifying, managing, and controlling their use. While organisms that have been highly modified by classical strain selection have been safely used in industry for years, there is a critical lack of data to either support or to allay concerns about the release into the open environment of organisms that have been modified by recombinant DNA techniques. A substantial base of scientific information, monitoring methods, and predictive models is required, and chemical engineers have been working closely with the biology community to develop this base. The chemical engineering tools used to analyze chemical processes in industrial reactors have proven useful in analyzing the effect of releasing engineered microorganisms into the environment. Biodegradation is also a potential alternative to incineration for treatment of existing waste sites. Thus, for example, instead of digging up and trucking contaminated soil to an incinerator, this soil could instead be taken to a site where it is dumped into large quantities of water containing microorganisms selectively engineered to attack the specific waste components in the soil. Or instead, microorganism colonies could be injected directly into the effected soil at the waste site, allowing the soil to be decontaminated in place. There are tremendous technological and regulatory hurdles to overcome using the latter approach. It's already been mentioned that there are many as-yet unquantified issues associated with the release of non-native microorganisms into the environment. But even before that issue comes up, there is the question of whether the microbes can survive in the environment where they've been placed. It cannot be taken for granted that an organism bred and grown in a laboratory setting can be transferred to the open environment where there is little or no control of the surrounding temperature, oxygen level, water content, or nutrient availabilty. These very challenging engineering questions are under active investigation by chemical engineers. Separation processes Liquid-filled, porous, hollow-fiber membranes, such as the one shown in the figure below, hold promise for improving the efficiency and economics of extraction processes. In conventional liquid-liquid extraction, process design, hardware, and economics are dictated primarily by the relative densities of the two liquid phases. Energy must be expended to create a large surface area for diffusion to take place, while contact time may be shorter than desired because of high relative velocities of the two phases. Hollow fibers containing pores filled with the extracting agent permit the waste and stripping fluids to flow in a countercuffent fashion on opposite sides of the membrane. In this way, a high interfacial area can be maintained, regardless of the relative flow rate ol fluid to extracting agent. In addition, the extracting agent can be renewed by continuous desorption into the stripping fluid. This technique is but one example of many new processes evolving in the field of membrane separations. |
|
A liquid-filled ultrafiltration membrane. The
outer diameter of the cylinder is 100 microns (0.004 inch).
|
|
Another process for the separation of toxic chemicals from waste streams species involves adsorption from solution onto particles, followed by sedimentation (settling) to remove the toxic-laden particles. Solutes bound to the surface of aqueous particles may participate in oxidation-reduction reactions with the particles, undergo chemical transformations in which the particle surface serves as a catalyst (i.e., it facilitates the reaction), or participate in heterogeneous photochemical processes. The design of effective engineering processes for the treatment of water supplies to remove toxic compounds by adsorption/reaction/particle-removal sequences demands fundamental data on the kinetics of the individual steps and the incorporation of the data into process models. A major challenge is to describe all relevant chemical influences on the efficiency of removal of specific toxic compounds. Among these are the physical and chemical properties of the absorbing particle surface, the alteration of these properties by reactions or dissolution-precipitation processes, and the stability of aqueous particles to coagulation. In contrast to the chemical conditions of conventional municipal water and wastewater processing, the conditions selected or imposed by the special circumstances for control of hazardous substances may include extremes of pH (acidity), redox potential (oxidation ability), ionic content, and organic content. These factors may become critical in the design of optimal processes combining adsorption, reaction, and coagulation steps. Recent development of the use of reversed miceiles (aqueous surfactant aggregates in organic solvents) to solubilize significant quantities of nonpolar materials within their polar cores can be exploited in the development of new concepts for the continuous selective con- centrat ion and recovery of heavy metal ions from dilute aqueous streams. The ability of reversed micelle solutions to extract proteins and amino acids selectively from aqueous media has been recently demonstrated; the results indicate that strong electrostatic interactions are the primary basis for selectivity. The high charge-to-surface ratio of the valuable heavy metal ions suggests that they too should be extractable from dilute aqueous solutions. The potential of reversed micelles needs to be evaluated by theoretical analysis of the metal ion distribution within micelles, by evaluation of the free energy of the solvated ions in the reversed micelle organic solution and the bulk aqueous water, and by the experimental characterization of reversed rnicelles by small-angle neutron and x-ray scattering. Wet oxidation |
|
No, not according a report just released by the nonprofit Environmental Working Group and confirmed by the federal government's Environmental Protection Agency (EPA). The report states that, after almost 30 years, landmark Clean Water Act of 1972 is being thwarted by state pollution control agencies that let powerful polluters off the hook, and by federal officials who fail to step in. It goes on to say that 42% of state inspections of facilities that already had been found in violation of the Clean Water Act were "drive-by," in which inspectors were not required to get out of their cars. And more than 280 facilities run by known polluters were not inspected at all. These statements are based on data gathered by the EPA from October 1997 to October 1999. That 40% of America's water is still too polluted for swimming or fishing is all the more dramatic when one considers that the Clean Water Act carried the goal of making American waterways safe for those activities by 1983. Not that there hasn't been any progress since the Act went into effect. In 1972, approximately 60% of waterways were too polluted for swimming or fishing, and headlines were filled with environmental horrors: Ohio's garbage-choked Cuyahoga River bursting into flames; historic Boston Harbor called a cesspool; Lake Erie declared dead; and an oil spill blackening the scenic coast off Santa Barbara, California. Those environmental tragedies were largely righted. More broadly, an infusion of federal funds helped states improve sewage treatment plants so that raw—or barely treated—sewage no longer flows into lakes, rivers, streams, and bays. The law sharply reduced the flow of toxic chemicals that factory pipes were discharging directly into waterways, and raised standards for what constitutes clean water. But the growing problem of polluted runoff has offset gains. Rather than flowing from a point source such as a chemical plant, runoff in waterways protected by the Act comes from dirty, oily streets, construction sites, and farms and yards full of fertilizers and pesticides. |
|
Nearly 30% of the nation's industrial,
municipal, and federal facilities have been in serious
violation of the 1972 Clean Water Act in recent years,
dumping almost 270 million pounds of toxic substances into
U.S. waters in 1997 alone. The percentages represent the
fraction of facilities within each state that were in
significant non-compliance with the Act between October 1997
and December 1998. (Click on image for larger view.)
|
|
Although the Clean Water Act was passed because states were unable or unwilling to curb water pollution, the law has left enforcement largely to the states. Critics of government enforcement have little confidence that the states can solve today's problems, and want EPA to take a more active role. One consequence of state and local enforcement is that water quality varies widely from state to state. A fish one state considers too contaminated by mercury pollution to be eaten can be on the menu in another. The EPA's own Office of Inspector General criticizes the agency for allowing states to adopt weak water quality standards in violation of the Act. Today, many of the issues associated with ongoing pollution of the nation's waterways are political, not technical. It's essential that research continues on handling and managing toxic waste, but the fact is, much of what is enacted and enforced is the result of policy. |
|
|